Purpose: There is a large body of evidence supporting the efficacy of low level laser therapy (LLLT) also known as photobiomodulation (PBM) when used for the prevention and/or treatment of oral mucositis (OM) in patients undergoing radiotherapy for head and neck cancer (HNC). Recent advances in LLLT/PBM technology, together with a better understanding of mechanisms involved and dosimetric parameters may lead to the management of a broader range of complications associated with HNC treatment. This could enhance patient adherence to cancer therapy, and improve quality of life and treatment outcomes. This article discusses LLLT/PBM mechanisms of action, dosimetry and safety, and aims to identify the head and neck treatment side effects for which LLLT/PBM may prove to be effective. In addition, LLLT/PBM parameters for each of these complications are suggested and future research directions are discussed.
Methods: Narrative review and presentation of LLLT/PBM parameters based on current evidence and expert opinion.
Results: In vitro studies assessing the effect of LLLT/PBM on tumor cells have reported conflicting results. This diversity of effects is likely to be specific to LLLT power and dose. However, no clinical studies reported tumor protection or enhanced tumor growth as a result of LLLT exposure.
For OM management, optimal LLLT/PBM parameters were: Wavelength: typically between 633-685 nanometer (nm), or 780-830 nm; Energy density: laser or light-emitting diode (LED) output between 10-150 milliwatts; Dose: 2-3 Joules (J/cm2), and no more than 6 J/cm2 on the tissue surface treated; Schedule: 2-3 times a week up to daily; Emission type: continuous or pulsed ( A number of articles have suggested that LLLT/PBM may have utility in the management of other complications of treatment in HNC. To facilitate further studies, we propose potentially effective LLLT/PBM parameters for prophylactic and therapeutic use in supportive care for dermatitis, dysphagia, dry mouth, dysgeusia, trismus, necrosis, lymphedema, and voice/speech alterations.
Conclusion: LLLT/PBM may have a role in supportive care for a broad range of complications associated with the treatment of HNC with (chemo)radiation therapy. The suggested LLLT/PBM irradiation and dosimetric ranges, which are potentially effective for these complications, are intended to provide guidance for well-designed future studies. Although evidence suggests that LLLT/PBM is safe in HNC patients, vigilance remains warranted to detect any potential adverse effects of LLLT/PBM on cancer treatment outcomes and survival.
Low Level Laser Therapy/PhotoBioModulation (LLLT/PBM) for the management of orofacial and neck complications of cancer therapy
In the following paragraphs, we review selected acute and chronic complications associated with head & neck cancer (HNC) therapy, and the literature relevant to the use of LLLT/PBM for the management of these complications. For each complication, we propose prophylactic and therapeutic LLLT/LED protocols mainly based on evidence derived from the literature, and expert opinion (Table 2). These protocols are intended as clinical guidance and may serve as suggestions for continuing research.
Oral mucositis
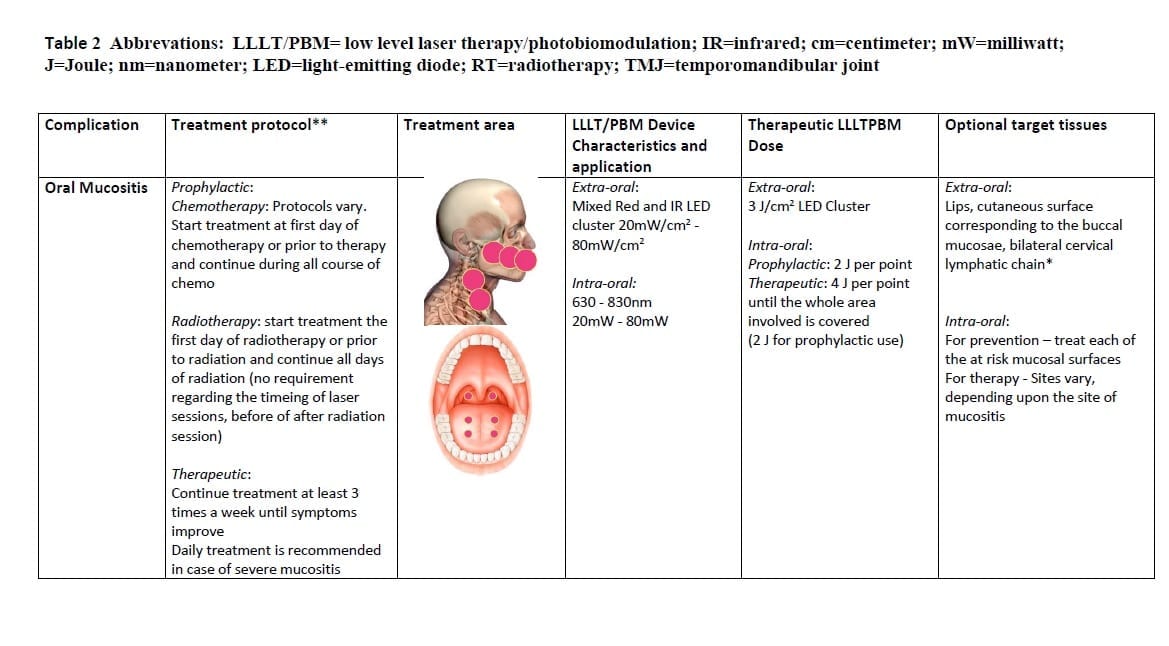 Oral mucositis affects virtually all patients undergoing CRT for HNC. Clinically, the manifestations of OM form a continuum, with erythematous mucosal changes when mild, and, ulcerative lesions that expose the submucosa when severe. The detrimental effects of OM upon QoL and functional status are significant [7].
Oral mucositis affects virtually all patients undergoing CRT for HNC. Clinically, the manifestations of OM form a continuum, with erythematous mucosal changes when mild, and, ulcerative lesions that expose the submucosa when severe. The detrimental effects of OM upon QoL and functional status are significant [7].
The current understanding of the pathogenesis of OM is largely based on animal models, which have shown the multifactorial nature of the condition and have implicated a cascade of interrelated events in multiple tissue compartments. These observations lead to the five-phase model of OM, based on the sequence of events following cytotoxic treatment [104]. The formation of excessive ROS and activation of NF-κB are the key factors in its pathobiology. Subsequent studies implicated microvascular injury, formation of proinflammatory cytokines, host–microbiome interactions, and extracellular matrix alterations in mucositis pathogenesis [105]. In addition, EGFR inhibitors and tyrosine kinase receptor inhibitors (TKI) administered as single drugs or combined with CRT may enhance OM or cause additional symptoms [5, 6]. Effective management options for OM are limited [106], and pain control is typically inadequate [7].
A Cochrane meta-analysis concluded that LLLT/PBM may prevent severe OM [11]. A systematic review and meta-analysis of 11 RCTs in HNC patients treated with CT and/or RT concluded that there was consistent evidence that LLLT/PBM applied with doses of 1–6 J per point reduced OM prevalence, severity and duration, and its associated pain [13]. Another meta-analysis including RCTs in various cancer treatment settings showed that LLLT/PBM reduced OM risk and decreased its severity and duration [14]. The efficacy appeared to be similar for red (630–670 nm) and NIR (780–830 nm) light, although the optimal doses seemed to vary between these wavelengths. Similarly, a systematic review and meta-analysis including 18 RCTs reported that prophylactic LLLT/PBM reduced severe OM and associated pain in patients treated for HNC or undergoing HSCT [16]. The Clinical Practice Guidelines of the Multinational Association of Supportive Care in Cancer and International Society for Oral Oncology (MASCC/ISOO) Mucositis Study Group found evidence for LLLT/PBM prevention of OM in patients undergoing HSCT, and patients treated with RT for HNC [15, 106]. Evidence was derived from high quality studies using specific LLLT/PBM parameters and the authors noted that there remains a need to identify optimal LLLT/PBM parameters per cancer type, and cancer treatment modality.
Based on this evidence and on our experience, we propose the following regimen for the management of OM (and mucositis affecting the oropharynx): wavelength of 633-685 nm, or 780-830 nm; power output of between 10 and 150 mW; Energy density 2-3 J/cm2, and no more than 6 J/cm2 on the tissue surface treated; frequency of 2-3 times a week up to daily; and using successive applications on single spots on a lesion rather than a scanning motion over the entire lesion. Emission type: continuous or pulsed.
Dermatitis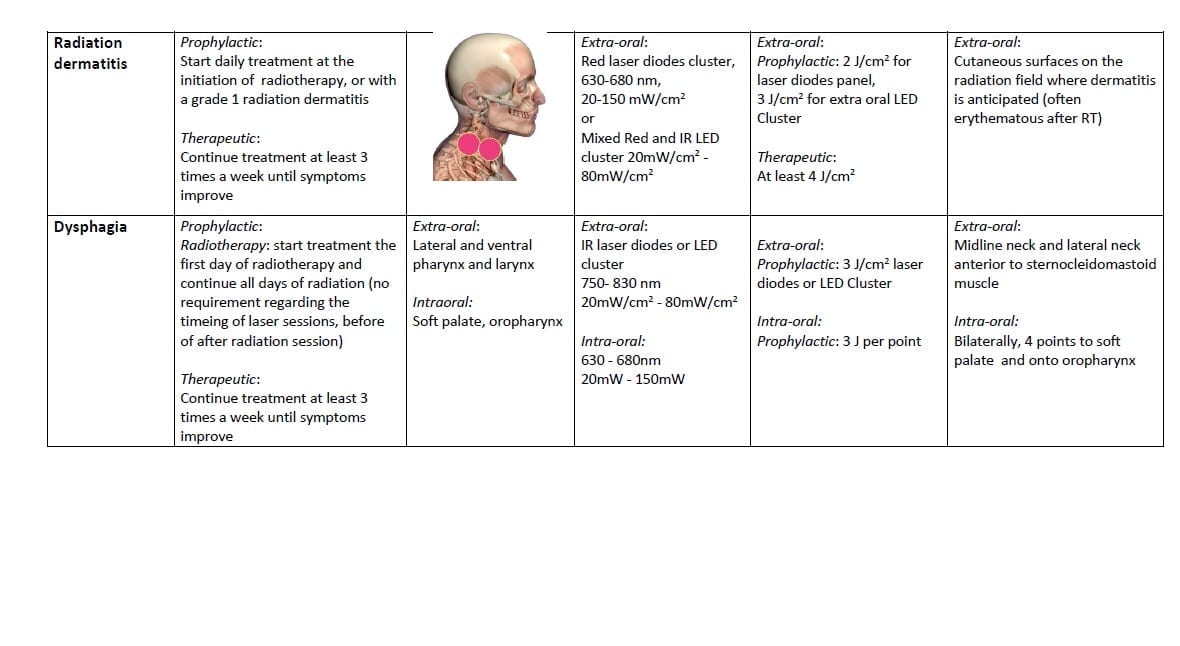
Radiation dermatitis occurs in the majority of patients with locoregionally advanced HNC treated with RT.
The pathobiology of acute radiation dermatitis is complex and partially overlaps that of OM. Irradiation of the skin leads to direct tissue injury and inflammatory cell recruitment, involving damage to epidermal basal cells, endothelial cells and vascular components [107]. Radiation-induced generation of free radicals induces DNA injury and release of inflammatory cytokines (mainly IL-1 and IL-6) [108, 109]. This process leads to development of erythema, edema and possible ulceration. Late RT-induced changes involving skin are characterized by the disappearance of follicular structures, an increase in collagen and damage to elastic fibers in the dermis, and a fragile epidermal covering [110]. TGF-β is considered to play a central role in mediating RT-induced tissue fibrosis [51, 111, 112].
The severity of skin reactions is dependent on the total radiation dose, the dose per fraction, the overall treatment time, beam type and energy, the surface area of the skin exposed to radiation, the use of combined chemoradiotherapy with or without targeted therapies, and individual risk factors [108]. The severity of acute reactions has been shown to predict late effects. Radiation dermatitis impacts adversely on cosmesis and function, especially in patients who develop secondarily infected dermatitis , and reduces QoL [107].
Patients with SCC of the head and neck treated with an EGFR- inhibitor may develop an acneiform skin rash in addition to radiation dermatitis [6, 110].
Based on the effects of LLLT/PBM on the epidermis and dermis (reduced inflammation and improved wound healing), and on the shared similarities in pathobiology with OM, it is reasonable to assume that LLLT/PBM may reduce the severity and/or prevalence of radiation dermatitis [113, 114] A study in pigs suggested that multi-wavelength LLLT/PBM ameliorated the development of late radiation damage to the skin [115]. DeLand et al [116] reported that LED treatments immediately after IMRT reduced the incidence of radiation dermatitis in patients with breast cancer, however, Fife et al [117] were not able to reproduce these results, although they did not specify important parameters such as irradiation time and size of area treated. A case series report described promising results for LLLT/PBM treatment at a NIR wavelength (970 nm) in patients with EGFR inhibitor-induced facial rash.
Dysphagia
Acute and chronic dysphagia (difficulty swallowing) and odynophagia (painful swallowing) are common in HNC patients, due to primary disease (oral, oropharyngeal, laryngeal and esophageal cancers) and those treated with RT or CRT [118, 119]. Dysphagia can be due to anatomical, mechanical or neurological changes affecting any structure from the lips to the gastric cardia [120]. Nguyen et al. [121] found 60% of patients to have dysphagia following CRT, of which 45% had severe dysphagia a median of 17 months post-treatment and chronic dysphagia is not likely to resolve over time [122].
Dysphagia associated with CRT has a complex pathogenesis, involving acute inflammation, edema, and fibrosis, with consequent neurological and muscular injury that may result in generalized weakness and a lack of muscle coordination while swallowing [123, 124] Excessive fibrosis results in a loss of elasticity that may contribute to chronic dysphagia [125, 126]. In addition, hyposalivation may contribute to dysphagia following CRT [8]. Moreover, the duration of total parenteral nutrition (TPN) or nasogastric tube feeding and resulting reduced swallowing, may affect the ability to return to safe, normal oral intake, since inactivity will cause atrophy of the swallowing muscles [127, 128]. Dysphagia negatively affects QoL [8, 129], and may predispose to aspiration and life-threatening pulmonary complications [122, 130].
Intensity modulated radiation therapy (IMRT) has emerged as an effective technique to deliver the full radiation dose to the tumor and regions at risk while reducing exposure of surrounding healthy tissues. Eisbruch and coworkers [131] identified dysphagia and aspiration risk structures (DARS) as susceptible to damage during IMRT. In particular, damage to the tongue base, pharyngeal constrictors, the larynx, and the autonomic neural plexus was found to be crucial in the development of post-RT dysphagia. Studies confirmed that reducing the radiation dose to DARS decreases dysphagia risk [132-134], but dysphagia remains a significant clinical problem [135].
The potential role of LLLT/PBM in the prevention and treatment of dysphagia requires further investigation. One study reported a lower incidence of severe OM and mucositis affecting the throat (contributing to acute dysphagia) when six predetermined oral sites were exposed to LLLT/PBM prior to and during RT [136]. In this study, dysphagia was scored indirectly by assessing the need for TPN. Given the ability of LLLT/PBM to prevent and ameliorate inflammation and pain associated with OM, and potential to control exuberant fibrosis [53], LLLT/PBM delivered to the DARS structures may have a potential role in the management of acute and chronic dysphagia. LLLT/PBM parameters for dysphagia are proposed in Table 2.
Hyposalivation and xerostomia
Another significant complication of RT to the head and neck region is hyposalivation, and its related complaint of xerostomia (subjective oral dryness). For all head and neck radiation regimens pooled, the weighted prevalence of xerostomia was found to be 6% before treatment, 93% during irradiation, and a slightly lower prevalence from one month to more than two years post-treatment [137]. Saliva plays an important role in maintaining mucosal integrity, promoting oral wound healing, taste perception, formation of food bolus, initiation of food ingestion, swallowing and speech [138]. Alterations in the oral microbiome, reduced oral clearance, changes in saliva composition (e.g., decreased buffer capacity, pH, immunoglobulin concentrations, defensins), and dietary changes may increase the risk for mucosal infections and rapidly progressing dental demineralization and caries [139]. A substantial decrease in salivary function has a significant impact on QoL, and results in an increased burden of long-term dental care [140, 141].
Irradiation of the salivary glands results in loss of gland function, beginning early in the course of RT [142] and has been shown to induce apoptosis in parotid glands in a dose-dependent manner. This process is p53-dependent [143]. There can be a modest improvement in xerostomia a few months after RT suggesting that an adaptation or compensatory function of non-irradiated salivary glands, or recovery of some of the function occurs. However, most patients have persisting oral dryness for the rest of their life, even when 3D conformal radiotherapy is used. With IMRT preserving more of the major salivary glands, long-term oral dryness may be reduced, but a significant proportion of patients still experience xerostomia [144].
The literature on LLLT/PBM for the management of hyposalivation is limited. In a study involving a variety of non-cancer patients with xerostomia, LLLT/PBM was applied daily: extra-orally to the parotid and submandibular glands and intra-orally on the sublingual glands. A gradual increase in the stimulated salivary flow was found after LLLT/PBM compared to controls [145]. Similar results in non-cancer patients were reported by Vidović et al [146]. Animal studies have shown an increase in the number of duct epithelial cell mitoses, and stimulation to protein synthesis in submandibular glands following LLLT/PBM [147, 148]. Similarly, a study reported the use of LLLT/PBM to increase salivary flow rate and amylase activity in rat parotid glands [149]. These authors also performed a study in HNC patients and reported that LLLT/PBM given concurrently with RT could prevent hyposalivation and xerostomia and had an impact on the composition of saliva [150]. Less severe xerostomia was also reported following prophylactic LLLT/PBM in HSCT recipients [151], and in a small RCT in HNC patients treated with RT [152], and increased salivary flow was observed in LLLT/PBM-treated patients when compared to controls [153]. A recent study performed in HNC patients at least 6 months following conventional RT found no improvement of hyposalivation and xerostomia, likely due to irreversible acinar atrophy and fibrosis [154].
These results point to the potential use of LLLT/PBM for prevention of hyposalivation and xerostomia; LLLT may also show efficacy for the treatment of hyposalivation when there is residual gland function following current RT modalities (parameters suggested in Table 2).
Taste alterations
Taste is one of the five senses and interacts with smell, touch and other physiological cues to affect the wider perception of flavor. Disturbed taste (dysgeusia) is complex and includes difficulties with smell and touch resulting in reduced food interest and affecting appetite and QoL. Taste function is the perception derived when food molecules stimulate taste receptors of the tongue, soft palate and the oropharyngeal region to perceive basic taste qualities (sweet, sour, salty, bitter and umami), that can be measured via standardized methods [155].
The prevalence of dysgeusia is estimated to be 66.5% following RT alone, and 76.0% after CRT; approximately 15% of patients continued to experience dysgeusia after treatment [156]. Ohrn and colleagues reported that the severity of taste alterations assessed by patients was correlated with the cumulative RT dose [157].
The mechanisms of dysgeusia during cancer therapy are not well understood: however, it is believed that CT and RT cause dysgeusia by destroying rapidly dividing taste bud cells and olfactory receptor cells [156]. Direct neurologic toxicity may also be involved, as taste recovery lags epithelial recovery and may continue indefinitely [158]. Hyposalivation may also have a significant contribution. The presence of the anterior part of the tongue in the radiation field may be predictive of taste disturbances [159].
Altered taste significantly affects overall QoL and may lead to energy and nutrient deficiencies and related complications and weight loss [8, 156]. Management options to decrease the prevalence and severity of taste problems are inadequate [158].
A pilot study reported that LLLT/PBM administered to taste buds may ameliorate neurologically mediated burning mouth syndrome symptoms including taste alterations [160], but to our knowledge, there are no published studies on LLLT/PBM for the management of taste problems in cancer patients. Hence, we feel that studies on the efficacy of LLLT/PBM for the management of dysgeusia in patients treated for HNC should be performed and propose potentially effective parameters in Table 2.
Trismus
Trismus refers to reduced opening of the jaws caused by spasm of the muscles of mastication, or may generally refer to all causes of limited mouth opening of less than 40 or less than 20 mm, whereas less restrictive classifications also have been used [161].
Limited mouth opening may be due to tumor, local infection, tissue fibrosis, pain upon mouth opening, a tonic contraction of the muscles of mastication or intrinsic changes in the temporomandibular joint.
The weighted prevalence of trismus is estimated to be 25% following conventional RT, 5% following IMRT and 31 % for CRT [162]. Patients may have limitations in jaw opening associated with tumor invasion of the masticatory muscles or the temporomandibular joint, or may develop trismus following RT to these structures [161, 163]. Cumulative radiation doses above 60 Gy are more likely to cause trismus [164], while the inclusion of the lateral pterygoid muscles in the high dose fields appears to be the most decisive factor [165]. Trismus typically develops 3-6 months post RT, and frequently becomes a lifelong problem [163, 166].
Studies have demonstrated that fibrosis is an important initial event in RT-induced trismus. Additionally, there may be scar tissue from surgery, nerve damage, or a combination of these factors [163]. Mandibular hypomobility ultimately results in muscle contraction and potentially temporomandibular joint dysfunction [162].
Trismus and orofacial pain interfering with function may have significant health implications including reduced nutritional intake, difficulty speaking, compromised oral health and poor QoL [167]. Aside from avoiding RT to the masticatory structures, early interventions are indicated to prevent or minimize trismus [3, 134, 168].
Concerning muscle spasms following oral surgery, a reduction was found in several studies using LLLT/PBM [169, 170]. To our knowledge, LLLT/PBM to prevent or reduce the severity of RT-induced trismus in HNC patients has not been reported. The potential of LLLT/PBM to reduce fibrosis and to promote muscle regeneration forms the main rationale for a potential clinical benefit. LLLT/PBM parameters are proposed in Table 2.
Soft tissue necrosis and osteoradionecrosis
Soft tissue and/or osteoradionecrosis (ORN) may occur as a consequence of RT. ORN is a process of radiation-induced vascular occlusion leading to loss of osteocytes and bone necrosis following RT [171]. The incidence of ORN has declined with proper pre-treatment dental care and advances in RT; in conventional RT, mandibular ORN prevalence ranges from 5%-15% but in the era of IMRT, less than 5% of patients are affected [163, 172].
The pathogenesis of ORN is not completely understood. It has been proposed that ORN occurs following a radiation-induced fibroatrophic process, including free radical formation, endothelial dysfunction, inflammation, microvascular thrombosis, fibrosis and remodelling, and finally bone and tissue necrosis [173]. Common triggers of necrosis are inflammatory dental disease, trauma to soft tissue, and dental surgical procedures in sites of high dose radiation exposure to bone. Removing diseased teeth after RT is considered a critical risk factor for ORN, but the lesion can also emerge due to periodontal disease, trauma or spontaneously [174-176]. Prevention of ORN is mainly based on extractions of compromised teeth before RT and adequate dental care during and following cancer therapy [1, 171].
Over the last years, angiogenesis inhibitors have been introduced in the treatment of advanced HNC [177]. Bevacizumab, an antibody that blocks VEGF may induce jaw osteonecrosis possibly as a result of tissue ischemia [178-180]. Bevacizumab may impair wound healing and can cause oral mucosal breakdown and exposure of necrotic jawbone [181]. Sunitinib, a tyrosine kinase inhibitor that blocks several pathways central to angiogenesis and tumor cell proliferation, has also been associated with osteonecrosis of the jaw [182, 183].
LLLT/PBM has a biostimulation effect on irradiated rat bone when applied before and during RT [184], and similar results were reported by El-Maghraby et al [185]. However, an in vivo study found that LLLT/PBM was not able to reverse RT-induced bone damage [186]. To our knowledge there are no clinical studies on the effects of LLLT/PBM for RT-induced jaw osteonecrosis. However, multiple studies suggest there was a benefit from LLLT/PBM in the management of bisphosphonate-related osteonecrosis of the jaw (BRONJ) [187-189]. Vescovi et al proposed a prophylactic protocol including LLLT/PBM for reducing BRONJ incidence following tooth extractions [190]. A study in a rodent wound healing model found evidence that both laser and LED were capable of stimulating angiogenesis in vivo [191]. These findings point to a possible role of LLLT/PBM in the management of jaw osteonecrosis induced by RT or angiogenesis inhibitors. We propose the use of LLLT/PBM parameters towards prevention and treatment of soft tissue and bone necrosis in future clinical trials in Table 2.
Head and neck lymphedema
A commonly neglected late effect in patients treated for HNC is secondary lymphedema [192], although these complications may be reduced with IMRT. Patients may develop lymphedema externally, on the face and neck, and/or internally involving the larynx and pharynx. External lymphedema may have a profound effect on appearance and body image [193], whereas internal lymphedema may impact breathing, contribute to dysphagia and trismus and may affect speech [194].
In a single center study on 81 HNC patients, 75% had lymphedema. Of those, 10% had external, 39% had internal, and 51% had both types of lymphedema [194]. Individuals with pharyngeal carcinoma were at highest risk [195]. Lymphedema typically develops 2-6 months after the completion of RT and may resolve spontaneously in some patients, but not in all. Assessment and measurement of head and neck lymphedema remains challenging [196].
Lymphedema is initiated by disruption of lymphatic structures by surgery or RT, resulting in the accumulation of lymph fluid in the interstitial tissues. This leads to infiltration of inflammatory cells and, because of the lymphatic dysfunction, cytokines and chemokines remain in the tissue and recruit additional inflammatory cells from the circulation. This ongoing inflammatory response results in additional soft tissue damage and fibrosis, which further adversely affects lymphatic function [197].
Low level laser therapy has been identified as a potential treatment for post-mastectomy lymphedema, as it stimulates lymphangiogenesis, enhances lymphatic motility, and reduces lymphostatic fibrosis [198]. Patients received additional benefits from LLLT/PBM when used in conjunction with standard lymphedema treatment [199]. Systematic reviews found evidence suggesting that LLLT/PBM reduced limb volume in patients with lymphedema following treatment for breast cancer [200-202].
It was concluded that future research should be performed comparing LLLT/PBM with standard practices and to establish the duration of laser application, number of treatment sessions, energy settings, power density, and dose. In addition, longer follow-up was considered necessary [201].
It has been proposed by Lee and coworkers that LLLT/PBM may also have a role in the management of lymphedema associated with HNC [203](Lee et al, 2013). Potentially effective LLLT/PBM parameters are summarized in Table 2.
Voice and speech alterations
Voice and speech are important communication tools and form part of a person’s identity and personality. Voice quality mainly depends on the movement and characteristics of the vocal cords, and speech on the resonance characteristics of the vocal tract. Speech is based on the volitional coordinated movements of the articulator structures and can be affected by any alteration in muscle or tissue properties of these structures. Although voice and speech dysfunctions significantly affect QoL, these complications have received little attention and are likely under-reported in efforts to preserve organ function after cancer therapy [204-206].
Currently, there is limited information on the prevalence of speech and voice dysfunction in advanced HNC patients treated with (C) RT and prospective studies are needed, including baseline measurements and standardized multidimensional assessment of functional aspects of voice and speech [205].
The etiology of voice and speech problems resembles that of dysphagia and may include neuromuscular weakness as a result of tumor invasion. CRT-induced voice and/or speech dysfunction can result from mucositis of the soft palate and laryngeal soft tissues, fibrosis or vocal fold atrophy, edema and atrophy of laryngeal and pharyngeal tissues and altered saliva or hyposalivation [207, 208].
New RT delivery techniques, including IMRT, designed to spare anatomical structures that are involved with voice and/or speech functions may prevent long-term functional impairment and early speech rehabilitation [209].
We are not aware of any studies on the effect of LLLT/PBM on the quality of speech and voicing in HNC patients. LLLT/PBM parameters for use in this complication is proposed in Table 2. They may preserve function of the anatomical structures involved directly, and could have indirect benefits by decreasing hyposalivation.
Conclusion
Acute and chronic complications induced by RT and CRT in patients with HNC represent a significant clinical challenge [1]. There are similarities with respect to pathophysiology across different complications, and patients may suffer from multiple concurrent and interrelated problems. There is anecdotal evidence suggesting that the inflammation associated with acute complications is a harbinger for chronic complications. This observation suggests that preventive approaches starting before, and in the early phases of treatment with RT and CRT, may reduce not only the risk for developing acute problems, but may also have an impact on the risk for late complications.
LLLT/PBM has shown effectiveness in the management of OM, and elicits several potentially beneficial effects, including reduction of inflammation and pain, promotion of tissue repair, reduction of fibrosis, and protection and regeneration of nerves. Therefore, there is a clear motivation for the application of LLLT/PBM to treat a broad range of acute and chronic complications associated with RT or CRT in HNC patients. RCTs should be conducted to assess the feasibility and efficacy of LLLT/PBM for prophylactic and therapeutic management of the head and neck complications of cancer therapy.
We hope that this article will serve as the basis for establishing a platform for facilitating future collaborations among clinicians and researchers, which will then create firm scientific evidence for the use of PBM in patients with HNC. LLLT and/or LED protocols should be administered using parameters that are likely to affect the anatomic structures at risk. The parameters (including the wavelengths) we have proposed are largely based on evidence derived from studies using LLLT/PBM for the management of OM (typically 633-685 nm or 780-830 nm). However, trials directed to other (non-head and neck) indications for the use of LLLT/PBM suggest that a broader range of wavelengths (590-1064 nm) has efficacy for healing, and for reducing inflammation and pain. Future investigations should be conducted to better define optimal photobiomodulation parameters for each of the complications of HNC treatment. In addition, the ideal timing, and frequency of LLLT/LED administration should be determined, as well as how long PBM should be continued following the completion of cancer treatment. PBM parameters should be reported in detail. Validated outcome measures must be identified and employed to assess the effect of prophylaxis and therapy, from the time of diagnosis through active treatment and survival.
Despite the potential benefits and plausible safety of LLLT/PBM for supportive care in HNC patients, vigilance remains warranted. While the reported results of in vitro studies of LLLT/PBM on malignant cells vary, and clinical reports have shown little or no adverse reactions, there is a paucity of robust data regarding potential protection and promotion of tumor. Even less data are available on potential beneficial effects of LLLT/BPM by enhancing the efficacy of (C)RT or immunologic antitumor reactivity. It is thus imperative that further investigations be directed to elucidating the effects of LLLT/PBM on oncology treatment outcomes and to obtaining more insight into the mechanisms for the host-tumor responses to LLLT/PBM.
